Tablet Dissolution Testing
Enquire NowWe provide the widest range of automation for Tablet Dissolution testing.
Closed Loop Dissolution Testing
This option uses our IDIS software to control dissolution testers and spectrophotometers on standalone or networked workstation.
Dissolution tester drivers are available for Agilent, Distek, Hanson, Sotax, Erweka, Logan, Pharmatest; and spectrophotometer drivers for Agilent, Thermo, PGI, Perkin Elmer and Shimadzu.
Sample is pumped before the time interval and a reading is acquired from the spectrophotometer at Intervals configured by the user. The dissolution result is calculated and displayed on a local workstation or on a networked WAN workstation where it can be accessed anywhere in the world in real time.
Professional Informative and colourful reports are easily configured by dragging and dropping report objects (graph, data table etc.,) to create templates.
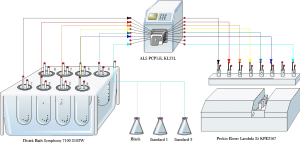
Products required for closed loop dissolution analysis:
-

IDIS Data Management Software
-

Pump PCP151F(DG6-8)
-

VS600 Series 10-Port Sampling Valve
-

DXi-80 8-Syringe Diluter Dispenser
-

Agilent 805 Dissolution Tester
-

Electrolab TDT8 Dissolution Tester
-

Hanson Vision Elite 8 Dissolution Tester
-

Sotax AT Dissolution Tester
-

Agilent Cary 60 UV
-

Jena 200 Plus
-

PGI T70+
-

PE Lambda 365
-

PE Lambda 465
-

Shimadzu UV1800
-

Shimadzu UV2600
Collection, Sample Preparation and UV Measurement Dissolution Testing
This dissolution testing option can be used for analysis that cannot be measured online, for example where sample concentration is high even when using small pathlength Flow Cells or when samples need to be prepared prior to analysis for UV or HPLC.
Sample are collected into Test Tubes which can be analytically diluted with dilution ratios as high as 1:30, or serially diluted to much higher dilution ratios.
After mixing, samples are injected into the spectrophotometer, Absorbance data is read and dissolution profile calculated in real time.

Products required for collection, sample preparation and UV measurement:
-

IDIS Data Management Software
-

ASP001 Dual Probe Single and Multiple
-

Diluter Dispenser DXI50
-

Pump PCP151F(DG6-8)
-

MSV2X800 Media Switcher
-

DXi-80 8-Syringe Diluter Dispenser
-

Agilent 805 Dissolution Tester
-

Agilent Cary 60 UV
-

Jena 200 Plus
-

PGI T70+
-

PE Lambda 365
-

PE Lambda 465
-

Shimadzu UV1800
-

Shimadzu UV2600
Collection, Sample Preparation and HPLC Measurement Dissolution Testing
We provide unique fully automated HPLC analysis when connected to Agilent ChemStation with 1100 and 1200 series HPLC’s or semi-automatically with other makes of HPLC.
Samples are collected into HPLC vials which can be further analytically diluted with dilution ratios as high as 1:30, or serially diluted to much higher dilution ratios.
After mixing, samples are injected into the HPLC.
With ChemStation, chromatography results are acquired, and the dissolution profile is calculated in real time.
The laborious task of creating Sample Set / Sequence Tables are manged by our IDIS software.
After creation, the Sample Set / Sequence Table are seamlessly transferred automatically to the HPLC CDS. IDIS also acquires peak results from the HPLC and plots the dissolution in real time.

Products required for collection, sample preparation and HPLC measurement:
-

IDIS Data Management Software
-

ASP001 Dual Probe Single and Multiple
-

Diluter Dispenser DXI50
-

Pump PCP151F(DG6-8)
-

VS600 Series 10-Port Sampling Valve
-

MSV2X800 Media Switcher
-

DXi-80 8-Syringe Diluter Dispenser
-

Agilent 805 Dissolution Tester
-

Electrolab TDT8 Dissolution Tester
-

Hanson Vision Elite 8 Dissolution Tester
-

Sotax AT Dissolution Tester
-

Chemstation Open Lab for Agilent 1100/1200 HPLC
Media Addition with pH Measurement Dissolution Testing
All dissolution configurations can have the option for Media Addition with or without pH measurement.
We can measure the pH in just 1 vessel or in all vessels and the pH values are numerically and graphically displayed in real time. Additionally, very specialised algorithms allows pH Titration to a set pH value. The Media Addition and pH Measurement option can be used with Closed Loop UV and with ASP2000 systems for UV or HPLC analysis

























